Photophysical and photodynamic analysis of different formulations of riboflavin
Medical hypothesis, discovery & innovation in optometry,
Vol. 4 No. 4 (2023),
25 December 2023
,
Page 181-187
https://doi.org/10.51329/mehdioptometry189
Abstract
Background: Riboflavin (Rb) has been used in the ophthalmological procedure known as corneal cross-linking (CXL). Pathologies requiring this treatment include keratoconus, corneal ectasia, and infectious keratitis. Rb is instilled via different molecules that are transported into the tissues. However, each vehicle imparts different properties that alter the photodynamic behavior of Rb, leading to variable concentrations of free radicals within the medium. The objective of this study was to measure the concentrations of free radicals produced by commonly used Rb formulations. To determine the free radical production level of each formulation, L-tryptophan (L-Tryp) was used as a model substrate because it can be efficiently photo-oxidized.Methods: We investigated the photodegradation of L-Tryp and its kinetics upon light exposure. The spectra were recorded using a Shimadzu UV-1800 PC spectrophotometer and a Cary Eclipse fluorescence spectrophotometer. A high-power solid-state LED light source was used for irradiation. L-Tryp degradation was performed using a 9-W LED lamp, and steady-state photolysis was conducted in quartz cells. The observed rate constants for L-Tryp degradation were determined by analyzing the changes in absorbance and fluorescence intensity. Data analysis was performed using Origin software.
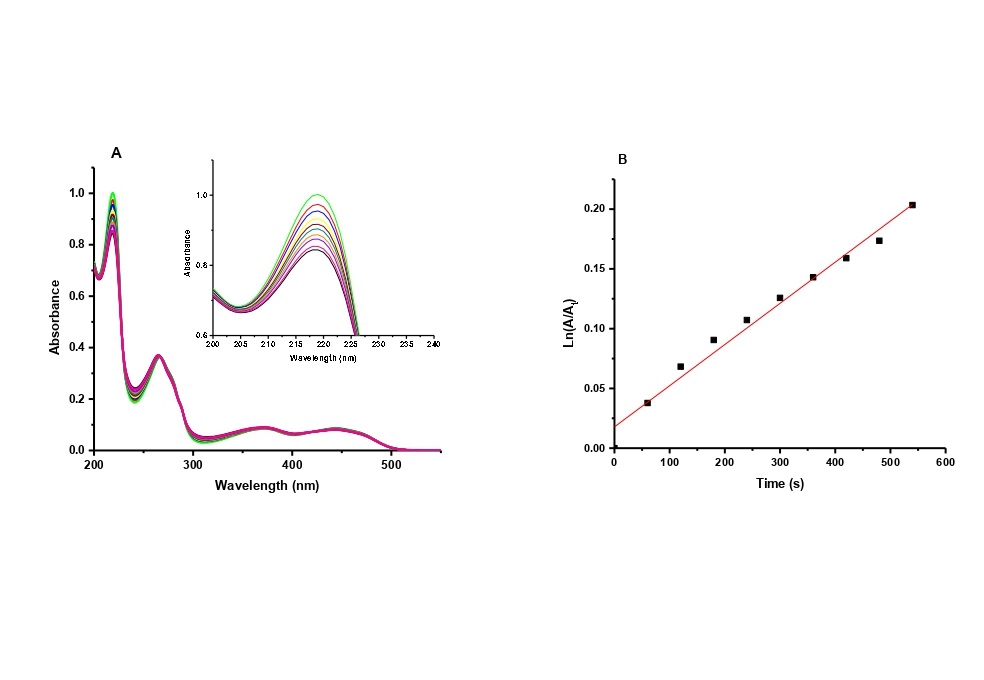
Results: We examined the characteristics of the photophysical and photodynamic action of the carriers in different commercially available Rb formulations. These included a) Rb with dextran, b) Rb without dextran, c) VibeX Rapid® (hydroxypropylmethylcellulose as a vehicle), d) Trans-Epithelial Kit (I) (sodium chloride as a vehicle), and e) Trans-Epithelial Kit (II) (benzalkonium chloride as a vehicle), using L-Tryp as a model substrate, and focusing on absorption and emission spectra. VibeX Rapid® exhibited the highest photo-degradation constant. The study affirmed the stability of Rb formulations for CXL and highlighted the efficacy of VibeX Rapid® in L-Tryp photo-oxidation and this rationalizes its current use as a CXL agent.
Conclusions: We demonstrated that formulations for transport of Rb are of crucial importance in CXL applications. Rb in the VibeX Rapid® formulation is more effective in generating photo-degradation, and this reflects its superior performance in CXL. Future experiments should be designed and conducted to quantitatively differentiate the production of free radicals. Studies involving human participants could shed light on the clinical efficacy and safety of the available Rb formulations.
Keywords:
- drug carrier
- corneal cross linking
- epi off CXL
- epi on CXL
- vitamin B2
- riboflavin
- L- tryptophan
- ultraviolet spectrophotometry
- fluorescence spectrophotometry
References
1. Hafezi F, Randleman JB. PACK-CXL: defining CXL for infectious keratitis. J Refract Surg. 2014;30(7):438-9. doi: 10.3928/1081597X-20140609-01 pmid: 24983827
2. Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2010;94(8):965-70. doi: 10.1136/bjo.2009.164228 pmid: 19666925
3. Wan KH, Ip CKY, Kua WN, Chow VWS, Chong KKL, Young AL, et al. Transepithelial corneal collagen cross-linking using iontophoresis versus the Dresden protocol in progressive keratoconus: A meta-analysis. Clin Exp Ophthalmol. 2021;49(3):228-241. doi: 10.1111/ceo.13918 pmid: 33667017
4. González Castellanos JC, Osaba M, Reviglio V, Canchi MT, Arrigone MC, Reviglio VE. Early treatment of bilateral fungal keratitis with corneal cross-linking as adjuvant therapy. Oxf Med Case Reports. 2020;2020(6):omaa032. doi: 10.1093/omcr/omaa032 pmid: 32551125
5. Rubinfeld RS, Caruso C, Ostacolo C. Corneal Cross-Linking: The Science Beyond the Myths and Misconceptions. Cornea. 2019;38(6):780-790. doi: 10.1097/ICO.0000000000001912 pmid: 30882538
6. da Paz AC, Bersanetti PA, Salomão MQ, Ambrósio R Jr, Schor P. Theoretical basis, laboratory evidence, and clinical research of chemical surgery of the cornea: cross-linking. J Ophthalmol. 2014;2014:890823. doi: 10.1155/2014/890823 pmid: 25215226
7. Messmer EM. Update on corneal cross-linking for keratoconus. Oman J Ophthalmol. 2013;6(Suppl 1):S8-S11. doi: 10.4103/0974-620X.122288 pmid: 24391374
8. Idrus EA, Utti EM, Mattila JS, Krootila K. Photoactivated chromophore corneal cross-linking (PACK-CXL) for treatment of severe keratitis. Acta Ophthalmol. 2019;97(7):721-726. doi: 10.1111/aos.14001 pmid: 30593737
9. Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473(4):347-64. doi: 10.1042/BJ20150942 pmid: 26862179
10. Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. Photosensitizers for Photodynamic Therapy. Adv Healthc Mater. 2019;8(13):e1900132. doi: 10.1002/adhm.201900132 pmid: 31067008
11. Oltulu R, ?atirtav G, Donbalo?lu M, Kerimo?lu H, Özka?nici A, Karaibrahimo?lu A. Intraoperative corneal thickness monitoring during corneal collagen cross-linking with isotonic riboflavin solution with and without dextran. Cornea. 2014;33(11):1164-7. doi: 10.1097/ICO.0000000000000249 pmid: 25211359
12. Mazzotta C, Traversi C, Caragiuli S, Rechichi M. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond). 2014;28(10):1179-83. doi: 10.1038/eye.2014.163 pmid: 25060847
13. Raiskup F, Pinelli R, Spoerl E. Riboflavin osmolar modification for transepithelial corneal cross-linking. Curr Eye Res. 2012;37(3):234-8. doi: 10.3109/02713683.2011.637656 pmid: 22335811
14. Lazzeri D, Rovera M, Pascual L, Durantini EN. Photodynamic studies and photoinactivation of Escherichia coli using meso-substituted cationic porphyrin derivatives with asymmetric charge distribution. Photochem Photobiol. 2004;80(2):286-93. doi: 10.1562/2004-03-08-RA-105 pmid: 15362952
15. Velazquez FN, Miretti M, Baumgartner MT, Caputto BL, Tempesti TC, Prucca CG. Effectiveness of ZnPc and of an amine derivative to inactivate Glioblastoma cells by Photodynamic Therapy: an in vitro comparative study. Sci Rep. 2019;9(1):3010. doi: 10.1038/s41598-019-39390-0 pmid: 30816179
16. Milanesio ME, Alvarez MG, Silber JJ, Rivarola V, Durantini EN. Photodynamic activity of monocationic and non-charged methoxyphenylporphyrin derivatives in homogeneous and biological media. Photochem Photobiol Sci. 2003;2(9):926-33. doi: 10.1039/b212890j pmid: 14560810
17. Reviglio VE, Osaba M, Sambuelli G, Kuo IC. Phototoxic Effect of Topical Fluoroquinolones Administered Before Corneal Crosslinking in a Murine Model. J Ocul Pharmacol Ther. 2017;33(2):73-78. doi: 10.1089/jop.2016.0060 pmid: 28106466
18. Mesen A, Bozkurt B, Kamis U, Okudan S. Correlation of Demarcation Line Depth With Medium-Term Efficacy of Different Corneal Collagen Cross-Linking Protocols in Keratoconus. Cornea. 2018;37(12):1511-1516. doi: 10.1097/ICO.0000000000001733 pmid: 30157054
19. Jain V, Gazali Z, Bidayi R. Isotonic riboflavin and HPMC with accelerated cross-linking protocol. Cornea. 2014;33(9):910-3. doi: 10.1097/ICO.0000000000000188 pmid: 25014154
20. Zaheer N, Khan WA, Khan S, Khan MAM. Comparison of Changes in Central Corneal Thickness During Corneal Collagen Cross-Linking, Using Isotonic Riboflavin Solutions With and Without Dextran, in the Treatment of Progressive Keratoconus. Cornea. 2018;37(3):340-346. doi: 10.1097/ICO.0000000000001496 pmid: 29283924
21. Velpandian T. Intraocular penetration of antimicrobial agents in ophthalmic infections and drug delivery strategies. Expert Opin Drug Deliv. 2009;6(3):255-70. doi: 10.1517/17425240902798119 pmid: 19327043
22. Ehmke T, Seiler TG, Fischinger I, Ripken T, Heisterkamp A, Frueh BE. Comparison of Corneal Riboflavin Gradients Using Dextran and HPMC Solutions. J Refract Surg. 2016;32(12):798-802. doi: 10.3928/1081597X-20160920-03 pmid: 27930789
23. Hagem AM, Thorsrud A, Sandvik GF, Drolsum L. Randomized Study of Collagen Cross-Linking With Conventional Versus Accelerated UVA Irradiation Using Riboflavin With Hydroxypropyl Methylcellulose: Two-Year Results. Cornea. 2019;38(2):203-209. doi: 10.1097/ICO.0000000000001791 pmid: 30365412
24. Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin Exp Ophthalmol. 2016;44(1):8-14. doi: 10.1111/ceo.12571 pmid: 26140309
25. Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54(2):1176-80. doi: 10.1167/iovs.12-11409 pmid: 23299484
26. Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The Biomechanical Effect of Corneal Collagen Cross-Linking (CXL) With Riboflavin and UV-A is Oxygen Dependent. Transl Vis Sci Technol. 2013;2(7):6. doi: 10.1167/tvst.2.7.6 pmid: 24349884
27. Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53(4):2360-7. doi: 10.1167/iovs.11-9385 pmid: 22427580
28. Fischinger I, Seiler TG, Wendelstein J, Tetz K, Fuchs B, Bolz M. Biomechanical Response After Corneal Cross-linking With Riboflavin Dissolved in Dextran Solution Versus Hydroxypropyl Methylcellulose. J Refract Surg. 2021;37(9):631-635. doi: 10.3928/1081597X-20210610-04 pmid: 34506235
29. Said DG, Elalfy MS, Gatzioufas Z, El-Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377-82. doi: 10.1016/j.ophtha.2014.01.011 pmid: 24576886
30. Hardwick CC, Herivel TR, Hernandez SC, Ruane PH, Goodrich RP. Separation, identification and quantification of riboflavin and its photoproducts in blood products using high-performance liquid chromatography with fluorescence detection: a method to support pathogen reduction technology. Photochem Photobiol. 2004;80(3):609-15. doi: 10.1562/2004-04-14-TSN-139 pmid: 15382964
31. Seghatchian J, de Sousa G. Pathogen-reduction systems for blood components: the current position and future trends. Transfus Apher Sci. 2006;35(3):189-96. doi: 10.1016/j.transci.2006.10.002 pmid: 17110168
32. Wainwright M, Baptista MS. The application of photosensitisers to tropical pathogens in the blood supply. Photodiagnosis Photodyn Ther. 2011;8(3):240-8. doi: 10.1016/j.pdpdt.2011.04.001 pmid: 21864797
33. Martin CB, Tsao ML, Hadad CM, Platz MS. The reaction of triplet flavin with indole. A study of the cascade of reactive intermediates using density functional theory and time resolved infrared spectroscopy. J Am Chem Soc. 2002;124(24):7226-34. doi: 10.1021/ja0123711 pmid: 12059249
34. Mazzotta C, Raiskup F, Hafezi F, Torres-Netto EA, Armia Balamoun A, Giannaccare G, et al. Long term results of accelerated 9?mW corneal crosslinking for early progressive keratoconus: the Siena Eye-Cross Study 2. Eye Vis (Lond). 2021;8(1):16. doi: 10.1186/s40662-021-00240-8 pmid: 33931101
35. Huvaere K, Cardoso DR, Homem-de-Mello P, Westermann S, Skibsted LH. Light-induced oxidation of unsaturated lipids as sensitized by flavins. J Phys Chem B. 2010;114(16):5583-93. doi: 10.1021/jp9121744 pmid: 20377218
36. Schiavi PC, Svetaz L, Petenatti E, Sortino M, Tempesti TC, Funes M. Extracts of Trichocline sinuata (Asteraceae) as natural sensitizers in the photodynamic inactivation of Candida albicans. Photochem Photobiol. 2023. doi: 10.1111/php.13871 pmid: 37877243
2. Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2010;94(8):965-70. doi: 10.1136/bjo.2009.164228 pmid: 19666925
3. Wan KH, Ip CKY, Kua WN, Chow VWS, Chong KKL, Young AL, et al. Transepithelial corneal collagen cross-linking using iontophoresis versus the Dresden protocol in progressive keratoconus: A meta-analysis. Clin Exp Ophthalmol. 2021;49(3):228-241. doi: 10.1111/ceo.13918 pmid: 33667017
4. González Castellanos JC, Osaba M, Reviglio V, Canchi MT, Arrigone MC, Reviglio VE. Early treatment of bilateral fungal keratitis with corneal cross-linking as adjuvant therapy. Oxf Med Case Reports. 2020;2020(6):omaa032. doi: 10.1093/omcr/omaa032 pmid: 32551125
5. Rubinfeld RS, Caruso C, Ostacolo C. Corneal Cross-Linking: The Science Beyond the Myths and Misconceptions. Cornea. 2019;38(6):780-790. doi: 10.1097/ICO.0000000000001912 pmid: 30882538
6. da Paz AC, Bersanetti PA, Salomão MQ, Ambrósio R Jr, Schor P. Theoretical basis, laboratory evidence, and clinical research of chemical surgery of the cornea: cross-linking. J Ophthalmol. 2014;2014:890823. doi: 10.1155/2014/890823 pmid: 25215226
7. Messmer EM. Update on corneal cross-linking for keratoconus. Oman J Ophthalmol. 2013;6(Suppl 1):S8-S11. doi: 10.4103/0974-620X.122288 pmid: 24391374
8. Idrus EA, Utti EM, Mattila JS, Krootila K. Photoactivated chromophore corneal cross-linking (PACK-CXL) for treatment of severe keratitis. Acta Ophthalmol. 2019;97(7):721-726. doi: 10.1111/aos.14001 pmid: 30593737
9. Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473(4):347-64. doi: 10.1042/BJ20150942 pmid: 26862179
10. Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. Photosensitizers for Photodynamic Therapy. Adv Healthc Mater. 2019;8(13):e1900132. doi: 10.1002/adhm.201900132 pmid: 31067008
11. Oltulu R, ?atirtav G, Donbalo?lu M, Kerimo?lu H, Özka?nici A, Karaibrahimo?lu A. Intraoperative corneal thickness monitoring during corneal collagen cross-linking with isotonic riboflavin solution with and without dextran. Cornea. 2014;33(11):1164-7. doi: 10.1097/ICO.0000000000000249 pmid: 25211359
12. Mazzotta C, Traversi C, Caragiuli S, Rechichi M. Pulsed vs continuous light accelerated corneal collagen crosslinking: in vivo qualitative investigation by confocal microscopy and corneal OCT. Eye (Lond). 2014;28(10):1179-83. doi: 10.1038/eye.2014.163 pmid: 25060847
13. Raiskup F, Pinelli R, Spoerl E. Riboflavin osmolar modification for transepithelial corneal cross-linking. Curr Eye Res. 2012;37(3):234-8. doi: 10.3109/02713683.2011.637656 pmid: 22335811
14. Lazzeri D, Rovera M, Pascual L, Durantini EN. Photodynamic studies and photoinactivation of Escherichia coli using meso-substituted cationic porphyrin derivatives with asymmetric charge distribution. Photochem Photobiol. 2004;80(2):286-93. doi: 10.1562/2004-03-08-RA-105 pmid: 15362952
15. Velazquez FN, Miretti M, Baumgartner MT, Caputto BL, Tempesti TC, Prucca CG. Effectiveness of ZnPc and of an amine derivative to inactivate Glioblastoma cells by Photodynamic Therapy: an in vitro comparative study. Sci Rep. 2019;9(1):3010. doi: 10.1038/s41598-019-39390-0 pmid: 30816179
16. Milanesio ME, Alvarez MG, Silber JJ, Rivarola V, Durantini EN. Photodynamic activity of monocationic and non-charged methoxyphenylporphyrin derivatives in homogeneous and biological media. Photochem Photobiol Sci. 2003;2(9):926-33. doi: 10.1039/b212890j pmid: 14560810
17. Reviglio VE, Osaba M, Sambuelli G, Kuo IC. Phototoxic Effect of Topical Fluoroquinolones Administered Before Corneal Crosslinking in a Murine Model. J Ocul Pharmacol Ther. 2017;33(2):73-78. doi: 10.1089/jop.2016.0060 pmid: 28106466
18. Mesen A, Bozkurt B, Kamis U, Okudan S. Correlation of Demarcation Line Depth With Medium-Term Efficacy of Different Corneal Collagen Cross-Linking Protocols in Keratoconus. Cornea. 2018;37(12):1511-1516. doi: 10.1097/ICO.0000000000001733 pmid: 30157054
19. Jain V, Gazali Z, Bidayi R. Isotonic riboflavin and HPMC with accelerated cross-linking protocol. Cornea. 2014;33(9):910-3. doi: 10.1097/ICO.0000000000000188 pmid: 25014154
20. Zaheer N, Khan WA, Khan S, Khan MAM. Comparison of Changes in Central Corneal Thickness During Corneal Collagen Cross-Linking, Using Isotonic Riboflavin Solutions With and Without Dextran, in the Treatment of Progressive Keratoconus. Cornea. 2018;37(3):340-346. doi: 10.1097/ICO.0000000000001496 pmid: 29283924
21. Velpandian T. Intraocular penetration of antimicrobial agents in ophthalmic infections and drug delivery strategies. Expert Opin Drug Deliv. 2009;6(3):255-70. doi: 10.1517/17425240902798119 pmid: 19327043
22. Ehmke T, Seiler TG, Fischinger I, Ripken T, Heisterkamp A, Frueh BE. Comparison of Corneal Riboflavin Gradients Using Dextran and HPMC Solutions. J Refract Surg. 2016;32(12):798-802. doi: 10.3928/1081597X-20160920-03 pmid: 27930789
23. Hagem AM, Thorsrud A, Sandvik GF, Drolsum L. Randomized Study of Collagen Cross-Linking With Conventional Versus Accelerated UVA Irradiation Using Riboflavin With Hydroxypropyl Methylcellulose: Two-Year Results. Cornea. 2019;38(2):203-209. doi: 10.1097/ICO.0000000000001791 pmid: 30365412
24. Ng AL, Chan TC, Cheng AC. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin Exp Ophthalmol. 2016;44(1):8-14. doi: 10.1111/ceo.12571 pmid: 26140309
25. Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54(2):1176-80. doi: 10.1167/iovs.12-11409 pmid: 23299484
26. Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The Biomechanical Effect of Corneal Collagen Cross-Linking (CXL) With Riboflavin and UV-A is Oxygen Dependent. Transl Vis Sci Technol. 2013;2(7):6. doi: 10.1167/tvst.2.7.6 pmid: 24349884
27. Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci. 2012;53(4):2360-7. doi: 10.1167/iovs.11-9385 pmid: 22427580
28. Fischinger I, Seiler TG, Wendelstein J, Tetz K, Fuchs B, Bolz M. Biomechanical Response After Corneal Cross-linking With Riboflavin Dissolved in Dextran Solution Versus Hydroxypropyl Methylcellulose. J Refract Surg. 2021;37(9):631-635. doi: 10.3928/1081597X-20210610-04 pmid: 34506235
29. Said DG, Elalfy MS, Gatzioufas Z, El-Zakzouk ES, Hassan MA, Saif MY, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377-82. doi: 10.1016/j.ophtha.2014.01.011 pmid: 24576886
30. Hardwick CC, Herivel TR, Hernandez SC, Ruane PH, Goodrich RP. Separation, identification and quantification of riboflavin and its photoproducts in blood products using high-performance liquid chromatography with fluorescence detection: a method to support pathogen reduction technology. Photochem Photobiol. 2004;80(3):609-15. doi: 10.1562/2004-04-14-TSN-139 pmid: 15382964
31. Seghatchian J, de Sousa G. Pathogen-reduction systems for blood components: the current position and future trends. Transfus Apher Sci. 2006;35(3):189-96. doi: 10.1016/j.transci.2006.10.002 pmid: 17110168
32. Wainwright M, Baptista MS. The application of photosensitisers to tropical pathogens in the blood supply. Photodiagnosis Photodyn Ther. 2011;8(3):240-8. doi: 10.1016/j.pdpdt.2011.04.001 pmid: 21864797
33. Martin CB, Tsao ML, Hadad CM, Platz MS. The reaction of triplet flavin with indole. A study of the cascade of reactive intermediates using density functional theory and time resolved infrared spectroscopy. J Am Chem Soc. 2002;124(24):7226-34. doi: 10.1021/ja0123711 pmid: 12059249
34. Mazzotta C, Raiskup F, Hafezi F, Torres-Netto EA, Armia Balamoun A, Giannaccare G, et al. Long term results of accelerated 9?mW corneal crosslinking for early progressive keratoconus: the Siena Eye-Cross Study 2. Eye Vis (Lond). 2021;8(1):16. doi: 10.1186/s40662-021-00240-8 pmid: 33931101
35. Huvaere K, Cardoso DR, Homem-de-Mello P, Westermann S, Skibsted LH. Light-induced oxidation of unsaturated lipids as sensitized by flavins. J Phys Chem B. 2010;114(16):5583-93. doi: 10.1021/jp9121744 pmid: 20377218
36. Schiavi PC, Svetaz L, Petenatti E, Sortino M, Tempesti TC, Funes M. Extracts of Trichocline sinuata (Asteraceae) as natural sensitizers in the photodynamic inactivation of Candida albicans. Photochem Photobiol. 2023. doi: 10.1111/php.13871 pmid: 37877243
- Abstract Viewed: 0 times
- Full Text PDF Downloaded: 0 times